Physicists Found A Novel Way To Peer Inside A Radioactive Molecule

Physicists Found A Novel Way To Peer Inside A Radioactive Molecule
Physicists have studied a rare molecule to look at how magnetism is distributed within a radioactive nucleus for the first time.
The rules of nature don’t, generally speaking, change. If you toss a ball in Seattle or in Tokyo, it falls the same way.
Physicists call this “symmetry”, and they use symmetry as a guide to how the universe ought to behave. It’s what keeps the world consistent — if the laws of physics worked differently on Tuesdays, the universe would be chaos.
the و a و to – تفاصيل مهمة
But some parts of nature don’t seem to follow this perfect balance. For example, it may seem fair to assume that the universe should treat matter and antimatter as equals. Yet our universe is madealmost entirely of matterand physicists still don’t know why.
One promising place to search for answers is inside radioactive nuclei. That is because the uneven arrangement of protons and neutrons can magnify the tiniest breaks in symmetry. If scientists are able to detect those small asymmetries, it could reveal new physics beyond the Standard Model, according toSilviu-Marian Udrescua physicist at MIT and co-author of a new study into the phenomenon.
the و to و is – تفاصيل مهمة
In a study published Oct. 23 in the journalSciencescientists at CERN and MIT examined a short-lived radioactive molecule called radium monofluoride (RaF) to measure its energy spectrum.
But, surprisingly, they ended up making the first observation of how magnetism is distributed within one of its nuclei. That phenomenon, known as the Bohr–Weisskopf effect, had never been seen in a molecule before.
The avocado of the atom
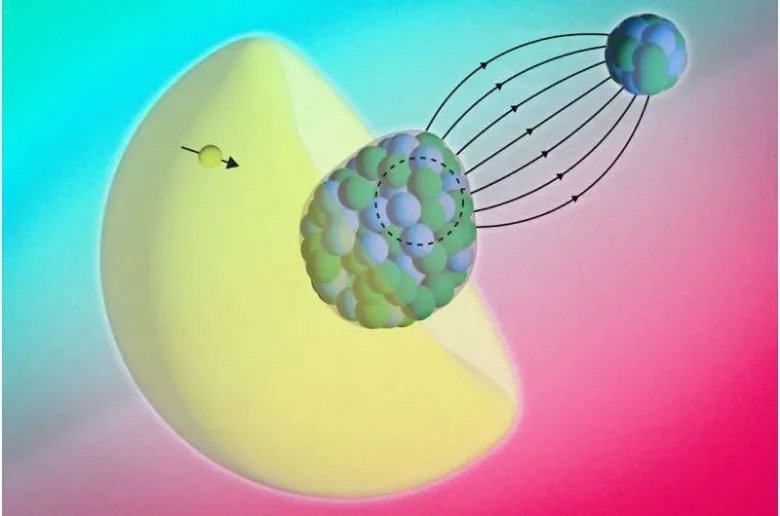
The RaF molecule is made of two atoms: radium and fluoride. Each with its own nucleus. The radium nucleus has a property called “octupole deformation”.
of و the و The – تفاصيل مهمة
“You can think of it as the nucleus itself having the shape of a pear or an avocado,”Shane Wilkinsa physicist at MIT and the study’s first author, told Live Science. Because of its asymmetric shape, RaF makes a perfect candidate to find the asymmetries the team was looking for.
“It’s a very rare property,” Udrescu added. “It only occurs in a few handfuls of atomic nuclei across the entire nuclear chart. And all of those nuclei that have this pear shape are radioactive.”
the و a و of – تفاصيل مهمة
That radioactivity makes such nuclei difficult to study because these isotopes are unstable and short-lived. That means they decay within around 15 days, and can disappear before researchers can make many measurements. “We can only produce them in very small quantities,” Wilkins said.
The Bohr-Weisskopf effect has been observed in individual atoms, where electrons interact with a single nucleus. However, detecting it inside a molecule is challenging. That is because electrons constantly move between the two nuclei.
The movement can blur magnetic signals and make them harder to detect. In a RaF molecule, the fluoride atom is a simpler bond partner. It allows scientists to focus on the magnetic structure of the heavier radium nucleus.
The team first created radium monofluoride atCERN’s ISOLDE facility. They blasted a uranium target with high-energy protons to produce the rare isotope radium-225 and combined it with fluorine gas.
Each molecule existed for only fractions of a second. The researchers could detect only about fifty per second in the right state for measurement.
Then, they directed multiple laser beams of slightly different frequencies at the molecules. When the molecule absorbed or emitted light, scientists recorded the tiny changes in that light. This produced a spectrum.
the و a و The – تفاصيل مهمة
Normally, those patterns tell scientists about how the electrons move around the nucleus. But in this case, some of the shifts revealed that the electrons were being influenced by the inside of the nucleus.
“The electron actually probesinsidethe nucleus, so you can no longer treat it as a long-range interaction. Instead, it starts to sense the internal properties of the radium nucleus itself,” said Wilkins.
“This effect is called the Bohr–Weisskopf effect,” Wilkins added. “To the best of our knowledge, it’s never been seen in a molecule before. The fact that we could both observe this effect experimentally and describe it with theory tells us a lot about how suitable these molecules are for future precision measurements.”
Now that the researchers have mapped RaF’s internal structure, they can use it to probe even smaller effects that might break nature’s symmetries. The next step, Wilkins said, is to slow and trap these molecules with lasers to perform even precise measurements.
the و it و to – تفاصيل مهمة
“Now we know they can be powerful tools to look for new physics,” said Udrescu.
p style=”font-size:18px;color:#555″>
Disclaimer: This news article has been republished exactly as it appeared on its original source, without any modification.
We do not take any responsibility for its content, which remains solely the responsibility of the original publisher.
p style=”font-size:14px;color:#555″>
Author:
Published on:2025-11-11 15:00:00
Source: www.livescience.com
Disclaimer: This news article has been republished exactly as it appeared on its original source, without any modification.
We do not take any responsibility for its content, which remains solely the responsibility of the original publisher.
Author: uaetodaynews
Published on: 2025-11-12 00:15:00
Source: uaetodaynews.com





